Part:BBa_K4359008
ClyA-V5-Affi
Introduction
ClyA-V5-Affi is the composite part derived from assembling Cytolysin A (BBa_K4359001), (SSSSG)x2 serine glycine linker (BBa_K4359003), V5 tag (BBa_K4359007) and anti-HER2 affibody (BBa_K4359002).
Biology
Cytolysin A acts as a localization signal and targets the protein to the bacterial outer membrane. Subsequently, outer membrane vesicles (OMVs) (here termed Affi-OMVs) derived from the outer membrane carry ClyA-V5-Affi. The serine-glycine linker is flexible and joins ClyA with the subsequent domains. The V5 epitope tag was added to enable antibody-based detection in Western blots and immunofluorescence. The anti-HER2 affibody is an affinity protein against the human epidermal growth factor receptor (HER2). Detailed information on each basic part can be found on their corresponding pages. This part is derived from ClyA-Myc-Affi (BBa_K4359006) by replacing the myc tag with the V5 tag. We found that anti-myc tag antibodies cross-react with components of mammalian cell lines and would be unsuitable for detecting ClyA-Myc-Affi in such a system. ClyA-V5-Affi was designed as an alternative.
This protein is designed to be displayed on the surface of bacterial outer membrane vesicles (OMVs) and target them to breast cancer cells overexpressing HER2. ClyA contains disulphide bonds and requires strains with periplasmic disulphide bond reduction machinery, such as dsba.
Usage
ClyA-V5-Affi was cloned into pGEX-4T1 between the EcoN1 and BamH1 sites. pGEX-4T1 is compatible with expression in K12 strains which lack a genomic copy of the T7 RNA polymerase (and hence cannot express proteins in an expression vector such as pET). The expression of ClyA-V5-Affi was under the control of the IPTG-inducible tac operon. We tested the expression with 1mM of IPTG.
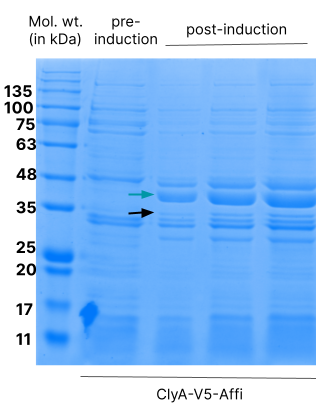
Affi-OMVs were isolated from E.coli cultures expressing ClyA-V5-Affi. This protein correctly localises to the OMV fraction. We confirmed this by a Western blot.

Previous Western blots indicate that the recombinant proteins are present in the OMV fraction but do not provide information about the orientation of the protein. For Affi-OMVs to be internalised, it is important for the affibody domain to be present on the external surface of the OMV, and not in the lumen or embedded in the membrane.
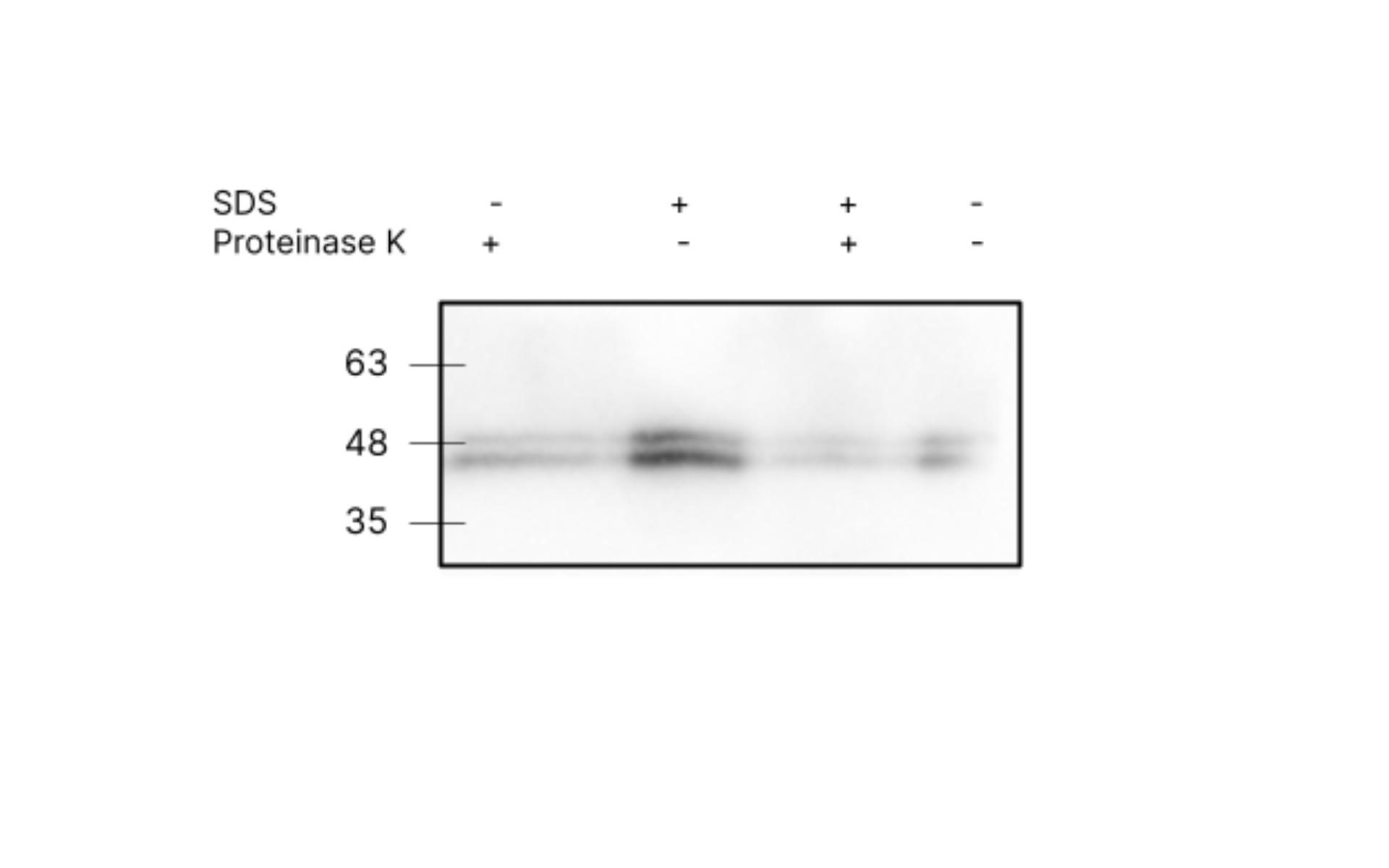
A proteinase K test was carried out for Affi-OMVs to investigate this. Proteinase K is a non-specific protease. If the affibody and the tag are exposed on the outer surface, we expect them to be degraded or truncated following Proteinase K treatment. If the affibody and tag are in the interior of the OMV, they should be protected from Proteinase K by the membrane, and should be intact. If OMVs are lysed with SDS prior to Proteinase K treatment, then regardless of the orientation, we expect protein degradation. The following results were obtained for this test:
The positive control (Proteinase K+, SDS +) failed in this experiment. We obtained inconclusive results.
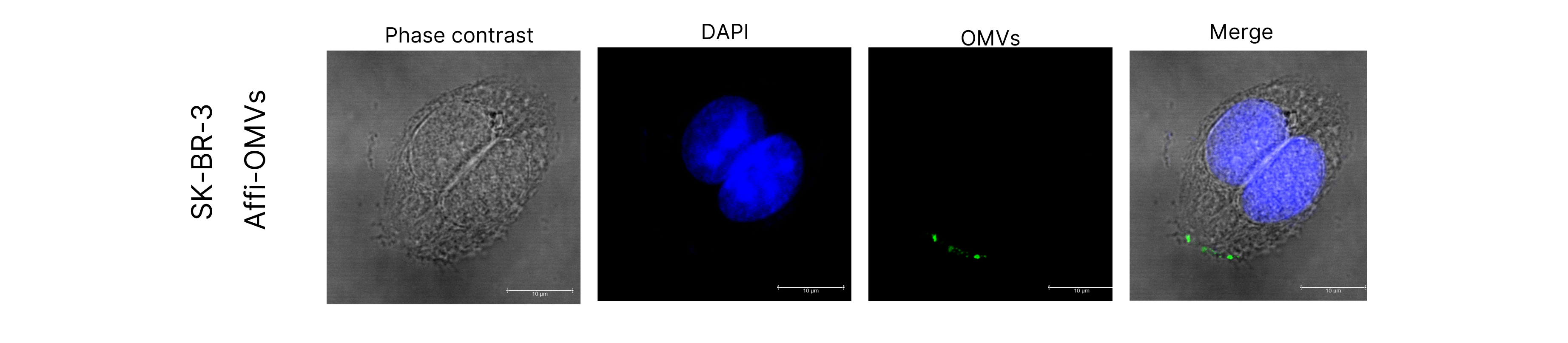
The ability of Affi-OMVs expressing ClyA-V5-Affi to selectively internalise in a cancer cell overexpressing HER2 is crucial. Affinity to HER2 is achieved by the affibody domain. We tested the uptake of Affi-OMVs in SK-BR-3, a HER2+ breast cancer cell line. Cells were incubated with 5ug of Affi-OMVs (quantity indicated is total protein concentration) for 4-5h at 37C. Cells were washed, fixed, stained and visualised by confocal microscopy.
We analysed the cell lysate following incubation of the above samples via Western blot.

We tracked the internalisation of Affi-OMVs ClyA-V5-Affi along with THP-OMVs expressing ClyA-3XFLAG-THP (BBa_K4359009). Together, Affi-OMVs and THP-OMVs comprise our system Duonco. For this experiment we co-incubated 5ug of Affi-OMVs and 5ug of THP-OMVs with SK-BR-3 cells for 4-5h at 37C. Cells were washed, fixed, stained and visualised by confocal microscopy.

We compared this to internalisation in HEK 293T, a cell line with comparatively low expression of HER2. A similar procedure was followed. We also tested the uptake of THP-OMVs. Cells were co-incubated with 5ug of Affi-OMVs and 5ug of THP-OMVs(quantity indicated is total protein concentration) for 4-5h at 37C. Cells were washed, fixed, stained and visualised by confocal microscopy.

We generally observed that in SK-BR-3 cells, Affi-OMVs were localised to the cell membrane or cell interior, while for HEK 293, Affi-OMVs remained as aggregates in the media and did associate with the cell membranes. This provides encouraging evidence for that ClyA-3V5-Affi targets OMVs for selective uptake in cells overexpressing the target marker HER2.
//chassis/prokaryote/ecoli
| None |